Modifications in the exposure of molecules at surface of abnormal cells, or those dying by apoptosis, ordinarily lead to their elimination by macrophages (phagocytosis). These changes are impaired in the tumor context and this can impede phagocytosis and cause resistances to therapies.
Using super-resolution fluorescence microscopy, also known as nanoscopy, because it can achieve a resolution of a few nanometers, researchers of the IBS/I2SR group have studied the distribution, diffusion and interactions of molecules (such as CD47, calreticulin, phosphatidylserine or the SIRPα receptor) known to induce or block phagocytosis. Their experiments suggest that disorganization of the lipid bilayer at the plasma membrane, by inducing inaccessibility of CD47, may have an influence on CD47/SIRPα interaction, an immune checkpoint well known to regulate phagocytosis. In particular, the cholesterol content of the cell membrane may play a central role in the phagocytosis process (high levels may inhibit phagocytosis, by promoting CD47-SIRPα interaction).
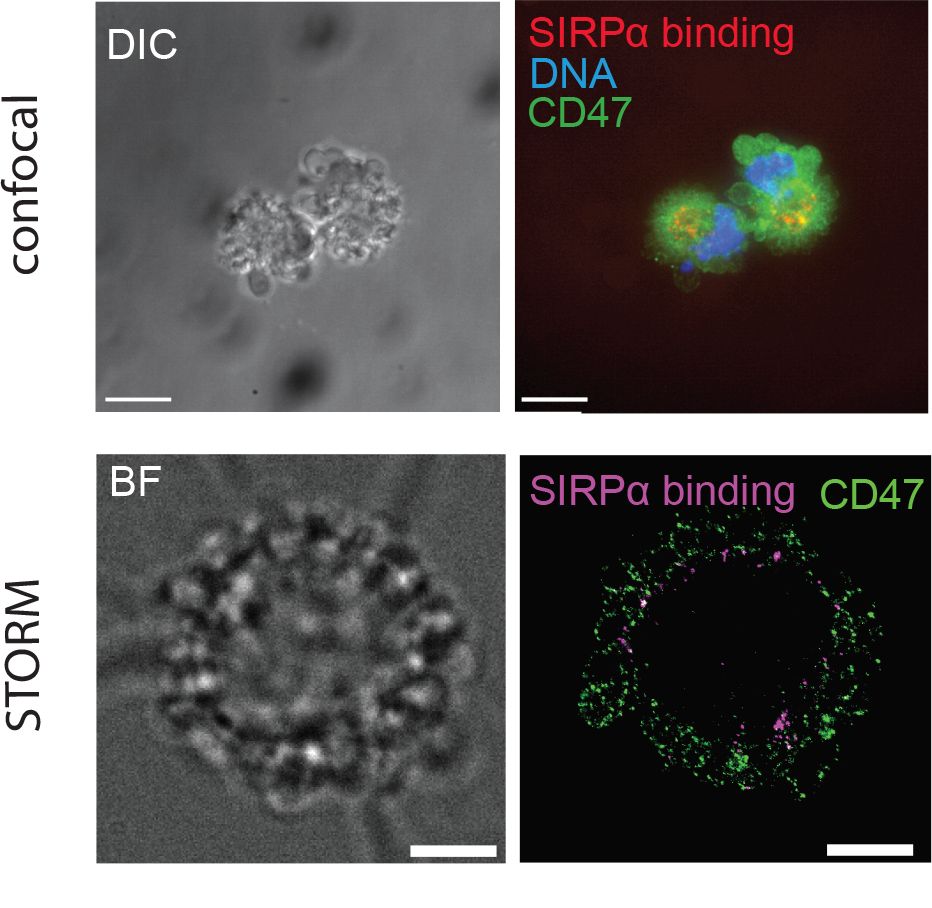
The increased resolution obtained thanks to STORM super-resolution microscopy
demonstrated that SIRPα is no longer colocalized with its CD47 ligand on the apoptotic blebs
This result opens up new perspectives for countering the mechanisms by which cancer cells escape the immune system.
Super-resolution imaging (STORM, Stochastic Optical Reconstruction Microscopy) and single-particle tracking (SPT) were carried out on instruments from the M4D platform at the Institut de Biologie structurale (member of ISBG, Integrated Structural Biology Grenoble).
Nanoscale imaging of CD47 informs how plasma membrane modifications shape apoptotic cell recognition. Samy Dufour; Pascale Tacnet-Delorme; Jean-Philippe Kleman; Oleksandr Glushonkov; Nicole Thielens; Dominique Bourgeois; Philippe Frachet. Communications Biology 2023; 6(1):207. doi: 10.1038/s42003-023-04558-y
Samy Dufour received funding from GRAL’s call for PhD operating costs.
